聊一聊血清学研究中的表面封闭剂
青木生物技术(武汉)有限公司
1705
Serologic assays are a very important method of detecting antibodies in the bodily fluids of patients and have a broad
applicability in confirming a patient’s immunological response to an infection or a vaccination. When new pathogens like
SARS-CoV-2 emerge and spread epidemically around the world, serologic testing is essential to identify currently infected
or potentially immune individuals1 . In addition, serological assays using the antigen-down format are routinely used in
autoimmunity and allergy testing. Surface blocking, which is crucial for all reliable immunoassays, is especially challenging
in antigen down formats and hence often causes problems and faulty results. Using ELISA as an example, we discuss the
reason for these problems, how to avoid them and how state-of-the-art surface blocking can help to improve many aspects
of modern serologic tests.
Surface blocking - only needed for low background?
Surface blocking - only needed for low background?
The surfaces of microwell plates are specifically treated to non-covalently bind all different kinds of proteins to allow for
high-density coating. However, this characteristic in turn causes the problem that many assay components, like antibodies
from the serum sample, can also nonspecifically bind to this surface, leading to faulty results. Good surface blocking prevents these unwanted binding reactions. Only the analyte can bind to the microwell surface via highly specific interactions with the coated capture molecules. In this context, good surface blocking means that all free binding capacities of the respective surface are saturated. This is achieved by the formation of a homogenous and very dense layer of blocking molecules with as few gaps as possible.
Unfortunately, the performance evaluation of blocking reagents is still too often very limited in scale and hence inappropriate for the significance of the result, which often guides the future treatment of an individual patient. An initial test to compare blocking reagents for the specific assay in question is the measurement of the signal of a sample that does not contain
the analyte (blank samples). Obviously, a higher signal, i.e. a higher background, in this preliminary test would immediately disqualify a specific blocking reagent. However, even if the blocking reagent performs well in terms of background intensity, this is only a first, but definitely not a sufficient step in the selection of the surface blocker during professional assay development. A much more discriminating and important test is the determination of the coefficient of variation of the background signal from a statistically relevant sample size. For the development and validation of serologic assays, there are a number of specialized suppliers that provide serum samples from donors with a variety of different disease backgrounds, like in.vent Diagnostica GmbH in Hennigsdorf/Berlin, Germany. Figure 1 illustrates how poorly a simple BSA surface blocker performs in terms of background signal variation in contrast to CANDOR’s The Blocking Solution in a simple comparison test using a competitive ELISA format. The mean background signal in the shown assay was indistinguishable.
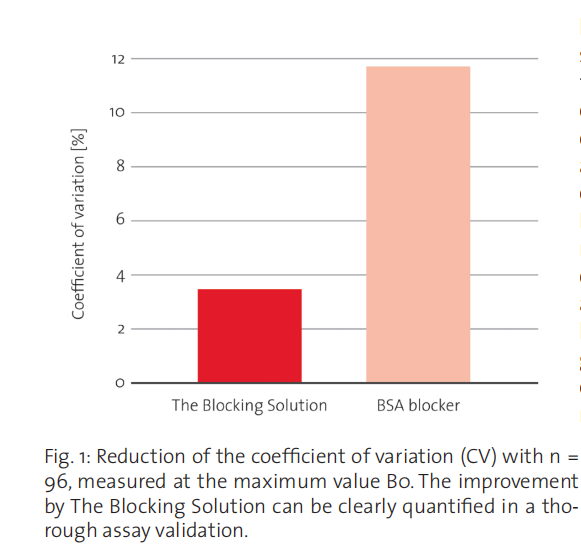

Together, a low background and a small coefficient of variation allow one to define a low detection limit2 , which is often set
three standard deviations above the mean background value.
The performance of a blocking reagent may also greatly depend on the specific assay in question and the exact chemical
composition of the surface, like the brand and type of microwell plate used for an ELISA. A surface blocker that performs well
in one may not be suitable for another assay due to differences in the assay format, capture molecule, surface properties or
the composition and physical properties of the specimens, as discussed below.
The selection of the samples used for the performance evaluation of the blocking reagent is also of great importance. It is
easy to achieve decent blocking results with non-problematic blank samples, like serum samples from young and healthy
individuals. This result is of little significance however, when the developed assay is later used to analyze the serum of patients with severe co-morbidities, for example cancer, autoimmune diseases like rheumatoid arthritis, or morbid obesity, as these specimen can often interfere with the blocking layer and cause faulty results.
As you can see, the selection and performance evaluation of a surface blocker is a difficult but important task that cannot
be solved with a one-buffer-fits-all solution if truly reliable assays are to be developed. This article is intended to give an
overview about common pitfalls in the selection of a surface blocker and how good surface blocking can greatly improve
assay quality. Problems that are frequently observed with respect to surface blocking are discussed in the following sections.
Covering of epitopes
Under certain circumstances, blocking reagents can interfere with the correct detection of analytes. This phenomenon is
rarely observed in sandwich assay formats with a capture antibody, mostly due to the large size of antibodies in general.
However, antigen-down assay formats - which are universally employed in serology - can encounter this problem, especially
if small capture antigens with a limited number of available epitopes are used. One example would be the approx. 25 kDa
Receptor-binding domain (RBD) of SARS-CoV-23 , which is the best-suited antigen for CoViD-19 immunity testing4. The most
common reason is a simple steric hindrance that blocks access of antibodies from the specimen to the capture antigen.
This can particularly occur if the blocking reagent mainly consists of molecules that are considerably larger than the coated
antigen, for example the commonly used protein BSA.
This would lead to false negative results, the classification of a sample as negative despite the analyte being present, and
hence is to be avoided. CANDOR’s SmartBlock™ is a surface blocker that prevents the described mechanism leading to the
covering of epitopes and is hence especially suited for use with small capture antigens. Based on chemically modified pepti
des, SmartBlock™ - in contrast to blocking reagents mainly containing larger molecules - is able to form a blocking layer that
also prevents smaller assay components from attaching to free binding sites on the surface and shows a batch-to-batch
consistency normally only achieved by synthetic blocking reagents.
Interactions of assay components with the blocking layer
Interactions of assay components with the blocking layer
Nonspecific binding events are a frequent reason for false results in immunoassays. In respect to surface blocking, different
types of nonspecific interactions can show up as increased background.
A common problem is that the detector antibody can nonspecifically bind either to a non-optimally blocked surface or, in
case the surface was saturated efficiently, to the blocking molecules themselves. This leads to false positive results since the
detector will be captured irrespective of the presence of the analyte. Sometimes nonspecific interactions can be mediated
by components of the sample that cross-link the detector antibody with the blocking layer. False positives due to interactions between the blocking layer and the detector have already been reported for commercially available SARS-CoV-2 serologic assays5,6. Detector antibodies are especially prone to these kinds of interference: on the one hand, the coupling of an enzyme, like HRP or AP, or a fluorophore can have an influence on the paratope of the antibody and influence its binding specificity. On the other hand, fluorophores are often very hydrophobic and are therefore prone to nonspecific interactions with equally hydrophobic parts of the blocking layer or assay components. While the described nonspecific binding or bridging of the detector antibody can be circumvented by using state-of-the-art assay diluents, like LowCross® HRP-Stab, that are designed to block interferences during detection, it is easy to prevent some of these effects by careful selection and performance testing of the blocking reagent.
BSA and casein are widely used blocking agents but face problems specifically in serologic testing. Because they are present
in many foods, some patients develop antibodies against these animal proteins. The presence of these antibodies manifests
itself in allergic reactions, mostly caused by antibodies of the IgE-isotype, but the presence of IgG antibodies has also been
described7,8. These human antibodies can then specifically interact with the blocking layer and cause persistent false positives in serologic testing that cannot be prevented by the use of HAMA-blockers or even modern interference-blocking assay diluents like LowCross-Buffer®.
Since these interactions are mainly caused by proteins, modern protein- and animal-free blocking reagents, like CANDOR’s
PlateBlock™, are a possible solution to the discussed false results.
Problems of blocking reagents
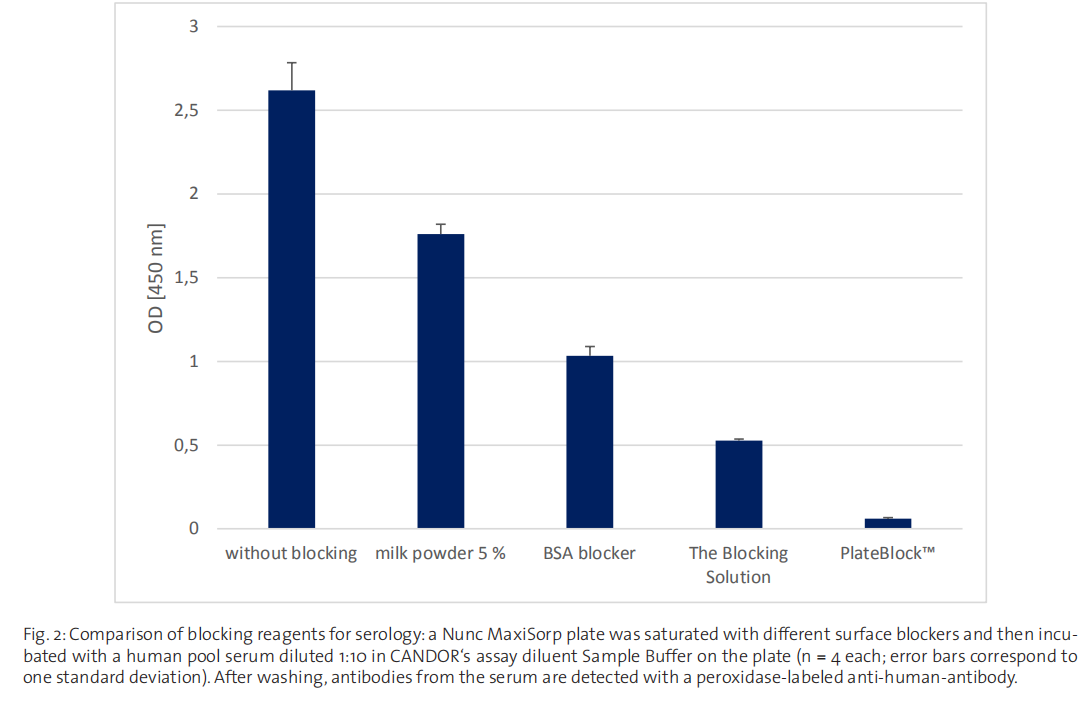
Exchange reactions with assay components
Efficient surface blocking is an important and complex topic to be solved. Yet, many assays employ poorly defined blocking
reagents, like milk powder, fetal calf serum (FCS), or fish extracts, to this day. A lot of university research is still conducted
using these blocking reagents, as witnessed by many, otherwise high quality research articles. With these reagents, elevated
background due to the presence of BSA or casein is the least of the problems that are to be expected. For one, especially
in serologic assays, milk powder performs poorly compared to modern blocking reagents (s. Fig. 2). This is to be expected,
since milk powder is a food product and was neither intended nor specifically designed for surface blocking, in contrast to
commercially available modern surface blocking reagents. Furthermore, being sourced from unprocessed animal products,
milk powder and FCS lack purity, i.e. they contain a plethora of different molecules that might interfere with the assay and
hence cause faulty results, and suffer from a high batch-to-batch variability. The former impacts the performance of the
assay, as it is unforeseeable how these impurities may impact the specific samples the end-user tries to analyze. The latter
is especially problematic in GLP laboratories or for the large-scale production of diagnostic assays. Every new batch of milk
powder or FCS poses the risk of variations that impact the reliability and performance of the assay, leading to high costs.
In extreme cases, some sensitive assays might require extensive re-validation of assay performance with every new batch
of milk powder or FCS. Lately with the EU regulation on in vitro diagnostics (IVDR), which will be in effect from May 2022,
these batch-to-batch variabilities will be problematic and pose the risk of failing to validate an assay that has taken a lot of
time and effort to develop. Short-term savings of only a few cents per sample may then lead to costly long-term problems.
The Blocking Solution from CANDOR solves all the problems these blocking reagents face. The Blocking Solution
is based on highly purified casein which has been chemically modified. The casein molecules are fragmented in a specific
procedure that results in a wide spectrum of variably sized fragments. This ensures an extremely dense packing structure
of the adsorptive blocking layer, while still showing a high batch-to-batch consistency. The chemical modifications preserve
these structures during the assay and minimize interactions. These properties are not only reflected in reduced background,
but also in an exceptionally low coefficient of variation (s. Fig.1). However, it is recommended for use in sandwich assays with
capture antibodies. For antigen-down assay one can do even better with modern serological surface blockers explained in
the following section.

Exchange reactions with assay components
Efficient blocking does not end with the saturation of all binding sites on the surface in the primary blocking reaction. The
blocking layer must also be stable, meaning the blocking reagent must prevent the detachment or replacement of individual molecules from the surface during the course of the assay. Otherwise, the freed-up binding sites can allow for the attachment of assay components, leading to false positive results. Especially in serology, it is striking that some patient’s serum and plasma samples tend to detach individual blocking molecules from a formerly dense layer, although most samples do not show this effect. While the exact mechanism of the detachment and exchange reaction is unclear, it ultimately leads to the binding of other molecules, mostly IgGs from the patient’s blood, to the surface. This results in elevated background values. Since these effects are highly dependent on the specific specimen in question, these problems may not be detected during short validations with low sample numbers and, as a consequence, patients with a serum that favors these exchange reactions will get false positive results. The issues are often circumvented by increasing the dilution of the sample to 1:100 or above, which helps to avoid the exchange reactions simply by the law of mass action (lower concentration of interfering molecules leads to fewer exchange reactions). However, lower sample dilutions of 1:10 to 1:50 allow for a much better limit of detection. This can be clinically relevant for example with serologic assays, because specific antibodies present in low concentrations may no longer be detectable. As is the case for SARS-CoV-2, some antibodies can have neutralizing activity and hence convey protection against infections even at titers below 10 ng/ml9,10. The detection limit is of great concern in the context of IgM or IgA assays for active SARS-CoV-2 infections where false negatives are to be avoided at all costs. Especially patients with asymptomatic or mild disease progressions often exhibit low antibody titers11,12.
Preventing these exchange reactions requires a very efficient blocking reagent that forms a very stable blocking layer. Commonly used blocking reagents, such as milk powder or BSA, are simply not up to this task in serological assays with low
sample dilutions of 1:10 (Fig. 2). Even CANDOR’s The Blocking Solution, while clearly superior to the standard reagents, is not
sufficient to adequately reduce background in this specific situation. For these challenging antigen-down assays CANDOR
has developed PlateBlock™, which was optimized to prevent the exchange reactions in the most comprehensive way by
forming a very resilient blocking layer on plastic surfaces (Fig. 2). In combination with LowCross-Buffer® or LowCross-Buffer®
STRONG as sample diluent, PlateBlock™ shows very good performance in serological ELISA tests, such as assays for neutralizing SARS-CoV-2 antibodies (s. also Fig. 5).
The many benefits of animal-free solutions
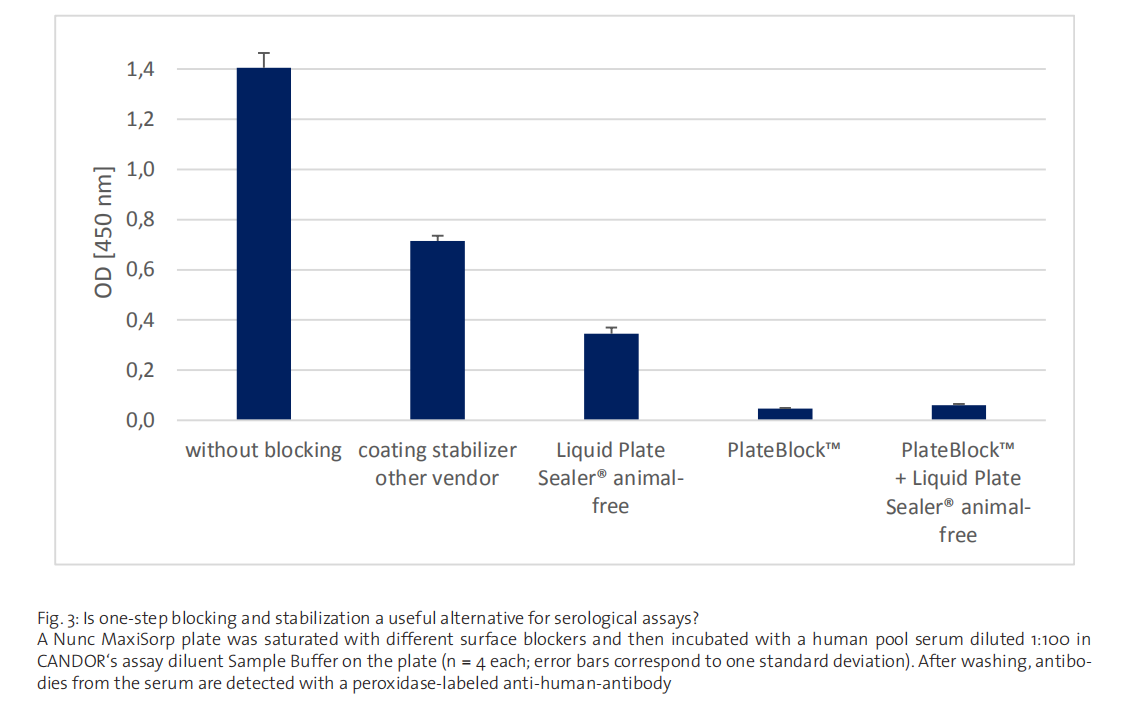
Animal-derived, protein containing blocking agents often face problems in modern diagnostic assays. As discussed earlier,
proteins from the blocking layer can be targets of patient antibodies or, due to the often promiscuous nature of protein
interactions, a source of unspecific binding, in both cases causing faulty assay results. Moreover, reagents like
milk powder or FCS lack purity and batch-to-batch consistency and often feature poorly defined contaminants. In food diagnostics, animal-derived blocking agents, like BSA or casein, may be especially problematic, as they can contain co-purifications of the target analyte or a closely related molecule, leading to the highly specific binding of the detector antibody to the blocking layer. These interferences cannot later be resolved using interference blocking assay diluents, like LowCross-Buffer®, as they can be caused by high affinity binding of specific antibodies.
A more administrative issue is that many countries have imposed strict regulations concerning the import of animal-by-pro
ducts13. Due to the risk of transmissible spongiform encephalopathies (TSEs), bovine-by-products, like BSA or casein, are often
under special scrutiny. This can make the international trade of products containing animal-by-products very bureaucratic
and time consuming. Moreover, stricter regulations on the manufacturing site will also inevitably lead to ever-increasing
costs of animal-derived components in the future, as can already be observed for FCS. In 2015 alone, the prices for FCS had
risen by 1400% and further increases are to be expected in the future14.
All of these issues can be avoided by using animal-free assay solutions. The increased demand has inspired CANDOR to
develop high-quality animal-free solutions for all immunoassay steps. Liquid Plate Sealer® animal-free serves to stabilize
antibodies and antigens after coating on polystyrene- or glass surfaces, thus allowing for long-term storage of dried ELISA
plates. Stabilization of plates is important if the coated molecules are unstable and lose their ability to capture the analyte in a few weeks, like the Receptor-binding domain of SARS-CoV-2 used in many serologic assays4. However, just like the
classic Liquid Plate Sealer®, its animal-free counterpart can also be used as surface blocker, which allows for blocking and
stabilization in a very convenient one-step process, thus saving time and production cost1 . This is often utilized in the manufacturing of classic sandwich ELISA plates. However, while being perfectly suited for many applications employing capture
antibody formats, these one-step processes without a separate blocking step are not suitable for serologic assays, due to
the aforementioned exchange reactions caused by serum samples. Despite not all European vendors being open about this
limitation, Liquid Plate Sealer® animal-free and other one-step solutions are outperformed by dedicated serology surface
blockers like CANDOR’s PlateBlock™ in this respect (Fig. 3). Yet, as shown, the combination of state-of-the-art surface blockers
and stabilizers allows for serologic assays that are excellently blocked and stabilized for long-time storage. PlateBlock™ and
Liquid Plate Sealer® animal-free in combination with CANDOR’s coating buffers, LowCross-Buffer® protein-free as sample
diluent, and HRP-Protector™ protein-free or LowCross® HRP-Stab protein-free as detector antibody diluents, enable the development and manufacturing of completely animal-free assays.

The advantages of specialized assay solutions
While the basic concept of ELISAs is simple to understand, mastering reliability and precision in ELISAs is a task that requires
a lot of theoretical background and experience. Despite the technology existing since the 1970s, developments in the last
decades have led to significant improvements in the automation of assay execution, the large-scale manufacturing, and the
performance of the required buffer solutions. Still, to this day, many scientists seem to think that the complexity of ELISA
development can be mastered with a simple one-buffer-fits-all solution, like the ever-present PBS-T/BSA. PBS-T/BSA is often
used for blocking, sample dilution and antibody dilution. Yet, like a compact-class car will neither outperform a sports car on
the racetrack, nor a truck in terms of cargo capacity, PBS-T/BSA cannot compete with the modern buffer solutions specifically
developed for each individual step of an ELISA. This can easily be illustrated when comparing the performance of PBS-T/BSA
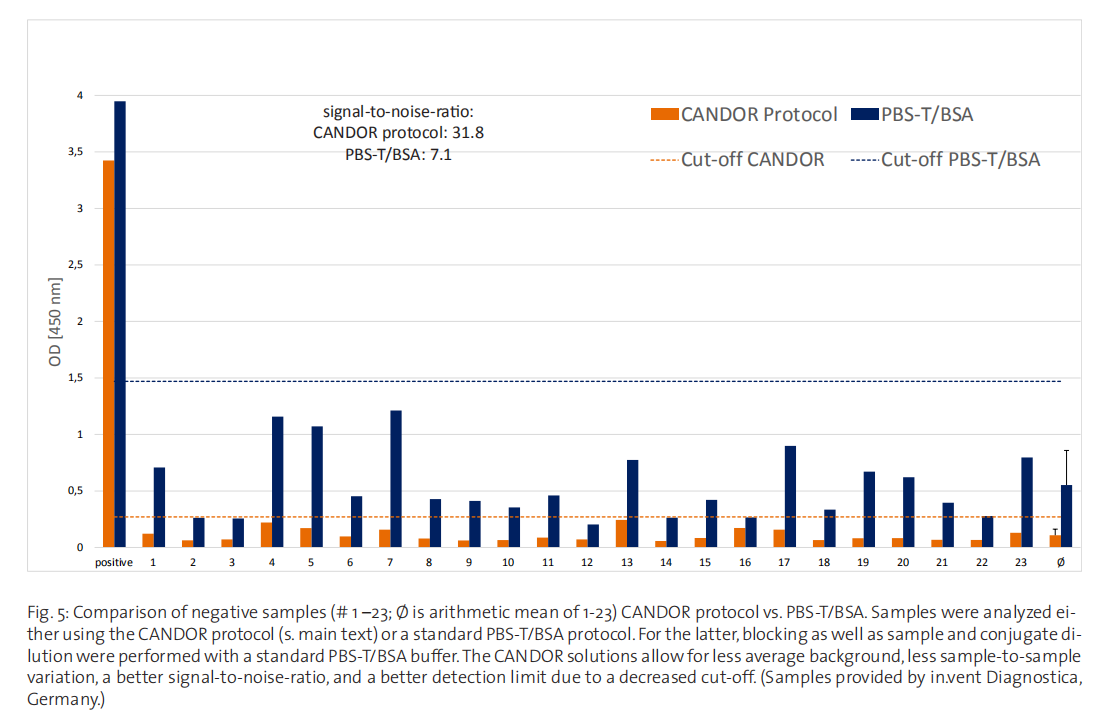
with more specialized solutions in serologic assays for antibodies against the RBD of SARS-CoV-2 (Fig. 5). While the signal
intensity in a positive sample is comparable, background from negative samples - serum samples taken before the CoViD-19
pandemic - is significantly lower using specialized buffer solutions, leading to a greatly improved signal-to-noise-ratio. Addi
tionally, the considerably reduced standard deviation for the CANDOR protocol means a lower cut-off can be defined, hence
lowering the detection limit and preventing faulty results.
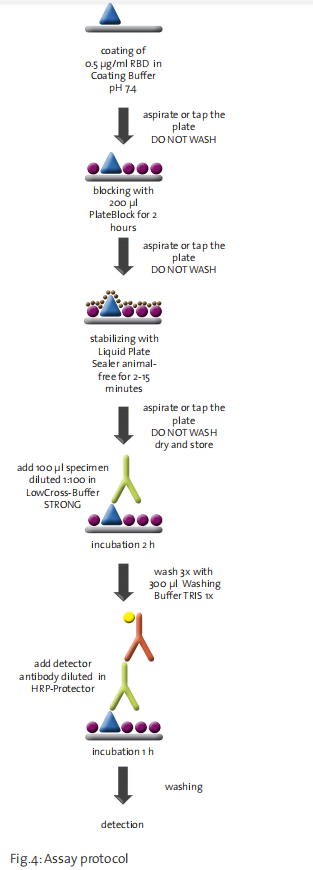
The following assay protocol was used:
1. Coating of 0.5 µg/ml RBD of SARS-CoV-2 (trenzyme, Germany) in Coating Buffer pH 7.4
2. Aspirate or tap the plate (do not wash)
3. Blocking with 200 µl PlateBlock™ for 2 h
4. Aspirate or tap the plate (do not wash)
5. Stabilization with Liquid Plate Sealer® animal-free for 2 - 15 min
6. Aspirate or tap the plate (do not wash), dry and store
7. Dilute the patient sample (1:100) in LowCross-Buffer® STRONG and incubate (2 h) 100 µl per well on the plate
8. Wash three times with 300 µl 1-fold Washing Buffer TRIS per well
9. Incubate (1 h) with detector conjugate stored in HRP-Protector™
10. Detect with substrate after further washing.

Notes:
• Avoid using detergents in all steps prior to application of the diluted sample when blocking with PlateBlock™
• If the plates are used directly after blocking, stabilization is not necessary and steps 5 and 6 can be omitted
Along with the described advantages of state-of-the-art surface blocking solutions, the results in figure 5 clearly show the superiority of specialized assay diluents in the development of modern immunoassays.
As discussed, even seemingly simple tasks, like the saturation of protein binding surfaces, requires a good understanding of the underlying mechanisms and specific blocking agents for different types of assays. Only with these modern solutions, reliable diagnostic assays will become the norm rather than the exception. One-buffer-fits-all concepts always lead to average quality, turning assays into failures in terms of reliability in every meaningful validation. Cost savings or even laziness must never go
at the expense of assay reliability and patient safety, much less so during a pandemic. For more than 15 years CANDOR Bioscience has successfully pursued the goal of progressively making immunodiagnostics and immunoassays for research more reliable. CANDOR Bioscience is always open to supporting all interested parties in the optimization of immunoassays, also with personal advice.

References
1: Polifke, T & Rauch, P (2020). The Covid-19 antibody test challenge. https://www.bionity.com
2: McNaught, AD & Wilkinson, A (1997) IUPAC. Compendium of Chemical Terminology, 2nd ed. Blackwell Scientific Publications, Oxford
3: Tai, W et al. (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol
4: Richter, SM et al. (2020) The challenges of serology - towards reliable SARS-CoV-2 antibody assays. https://www.biocompare.com/Future-Lab/Diagnostics/
5: Okba, NMA et al. (2020) Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus
Disease Patients. Emerging Infectious Diseases
6: Lassaunière, R et al. (2020) Evaluation of nine commercial SARS-CoV-2 immunoassays. Preprint at medRxiv
7: Chruszcz, M et al. (2013) Serum albumins - unusual allergens. Biochim Biophys Acta
8: Wal, JM (2004) Bovine milk allergenicity. Annals of Allergy, Asthma & Immunology
9: Robbiani, DF et al. (2020) Convergent Antibody Responses to SARS-CoV-2 Infection in Convalescent Individuals. Nature
10: Brouwer, PJM et al. (2020) Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability.
Preprint at bioRxiv
11: Lynch, KL et al. (2020) Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease
severity. Preprint at medRxiv
12: Marklund, E et al. (2020) Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG
non-responders. Preprint at medRxiv
13: Commission Regulation (EU) No 142/2011 of 25 February 2011
14: van der Valk, J et al. (2018) Fetal bovine serum (FBS): Past – present – future. ALTEX - Alternatives to animal experimentation