【共享】新一代的RNAi技术:非对称小RNA干扰
丁香园论坛
1875
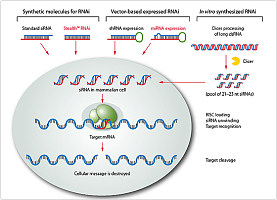
RNAi从发现到现在,短短几年时间,已经由一种生理现象发展为生物医学研究的重要手段和具有很大潜力的治疗手段。目前,哺乳动物细胞的RNAi主要依赖于长19~21bp,3‘端悬挂2nt的对称的RNA双链,即短干扰RNA(siRNA)。这里,作者报道了一种长15bp、3’和5‘端分别悬挂3nt的反义核苷酸的非对称的RNA双链,即非对称干扰RNA(aiRNA),可以有效诱导哺乳动物细胞RNAi的发生。研究表明aiRNA诱导的基因沉默高效、持久而且脱靶效应较低。因此,这也为我们在设计诱导哺乳动物细胞基因沉默的RNA双链结构时提供了一种新思路。
Asymmetric RNA duplexes mediate RNA interference in mammalian cells
Xiangao Sun1,3, Harry A Rogoff1,3 & Chiang J Li1,2
NATURE BIOTECHNOLOGY, 2008, 26:1379-1382
RNA interference (RNAi) has become an indispensable technology for biomedical research and has demonstrated the potential to become a new class of therapeutic. Current RNAi technology in mammalian cells relies on short interfering RNA (siRNA) consisting of symmetrical duplexes of 19–21 base pairs (bp) with 3’ overhangs. Here we report that asymmetric RNA duplexes with 3’ and 5’ antisense overhangs silence mammalian genes effectively. An asymmetric interfering RNA (aiRNA) of 15 bp was incorporated into the RNA-induced silencing complex (RISC) and mediated sequence-specific cleavage of the target mRNA between base 10 and 11 relative to the 5’ end of the antisense strand. The gene silencing mediated by aiRNA was efficacious, durable and correlated with reduced off-target silencing by the sense strand. These results establish aiRNA as a scaffold structure for designing RNA
duplexes to induce RNAi in mammalian cells.
Asymmetric RNA duplexes mediate RNA interference in mammalian cells
Xiangao Sun1,3, Harry A Rogoff1,3 & Chiang J Li1,2
NATURE BIOTECHNOLOGY, 2008, 26:1379-1382
RNA interference (RNAi) has become an indispensable technology for biomedical research and has demonstrated the potential to become a new class of therapeutic. Current RNAi technology in mammalian cells relies on short interfering RNA (siRNA) consisting of symmetrical duplexes of 19–21 base pairs (bp) with 3’ overhangs. Here we report that asymmetric RNA duplexes with 3’ and 5’ antisense overhangs silence mammalian genes effectively. An asymmetric interfering RNA (aiRNA) of 15 bp was incorporated into the RNA-induced silencing complex (RISC) and mediated sequence-specific cleavage of the target mRNA between base 10 and 11 relative to the 5’ end of the antisense strand. The gene silencing mediated by aiRNA was efficacious, durable and correlated with reduced off-target silencing by the sense strand. These results establish aiRNA as a scaffold structure for designing RNA
duplexes to induce RNAi in mammalian cells.