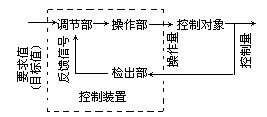
Tubuloglomerular Feedback
互联网
550
As originally described by Golgi, one consistent anatomical feature of all mammalian and many vertebrate kidneys is that a segment of the distal tubule is firmly attached to the vascular pole of its own glomerulum (1 ). This attachment is formed early in development, and is maintained throughout the elongation of the proximal convoluted tubule and the descending and ascending limbs of the loops of Henle. In mammalian kidneys, the tubular epithelium at the point of contact is differentiated into a specialized plaque of cells known as the macula densa (MD) cells. The MD cells, together with underlying interstitial cells and glomerular arteriolar smooth-muscle cells, form the juxtaglomerular apparatus (JGA). Another cell type in the JGA are the juxtaglomerular granular cells, modified smooth-muscle cells that are responsible for the synthesis and secretion of renin. The connection between tubule and glomerulum is the structural basis of a functional interaction known as tubuloglomerular feedback (TGF) (2 ). Whenever NaCl concentration at the MD deviates from its normal value, a signaling cascade is initiated that results in a dilatation of the vessel when the NaCl concentration decreases, and a constriction of the vessel when the NaCl concentration increases. Dilatation and constriction of afferent arterioles (AAs) is associated with increases or decreases in the single-nephron glomerular filtration rate (SNGFR) respectively, and these changes usually return MD NaCl toward normal.