流式细胞技术测定NF-kappaB的转移
丁香园论坛
A flow cytometry technique to study nuclear factor-kappa B (NFB) translocation during human B cell activation
Fabrice Cognasse a, Odile Sabido b, Lydie Béniguel a, Christian Genin a,c and Olivier Garraud a,c,d A olivier.garraud@univ-st-etienne.fr
Immunology Letters 2003, 90:49-52
[a] GIMAP-EA3064, Faculté de Médecine, Université Jean Monnet de Saint-Etienne, 15 rue Ambroise Paré, 42023 Saint-Etienne cedex 2, France Centre Commun de Cytométrie en Flux, Faculté de Médecine, Université Jean Monnet de Saint-Etienne, 15 rue Ambroise Paré, 42023 Saint-Etienne cedex 2, France[c] Laboratoire d'Exploration des Immunodéficiences, CHU de Saint-Etienne, France[d] Etablissement Français du Sang Auvergne-Loire, Saint-Etienne, France
A Corresponding author. Tel.: +33-4-77-81-4366; fax: +33-4-77-80-8294.
Manuscript received 11 May 2003 Accepted 20 July 2003;
Article Outline
Abstract
Introduction
Materials and methods
Cell preparations
Cell stimulation
Detection of NFB translocation by flow cytometric assay in CD19+ B cells nuclei preparations
Statistics
Results and discussion
Acknowledgements
References
Copyright
Abstract
We aimed at examining NFB translocation in B lymphocytes during in vitro activation through the specific receptor for antigen using a technique convenient in most laboratories such as flow cytometry. We present here an original, convenient, and reproducible technique to study B cell activation events through NFB translocation by means of a novel, specific flow cytometry assay. Intranuclear translocation of NFB p65 was induced after a 45 min stimulation; the highest signal was detected for a 10 ng/ml stimulus compared to the unstimulated condition (P<0.05). Purified CD19+ B cells—cultured in the presence of optimal concentrations of anti-µ fragment Abs (10 ng/ml) for 45 min at 37 °C—induced a mean 60% (range: 45–67%) MFI- and thus, nuclear NFB translocation-increase. We observed a one-pike profile of NFB staining in PBMC B cells and a two-pike profile of NFB staining in using tonsil B cells. B cells are susceptible to various dysregulations leading to minor to severe pathology (including immunoproliferative disorders). Studies of signal transduction carried out specifically in human B cells, using a novel technique gave considerable advantages: feasibility, sensitivity, reproducibility, ease.
Introduction
Nuclear factor kappa B (NFB) is a transcriptional factor involved in the expression of multiple target genes, the majority of which participate in the host immune response [1] including immunoglobulin (Ig) gene rearrangements [2,3] . Various types of stimuli (cytokines, CD40 ligation, TLR ligation, stress factors, microbial pathogens, etc.) activate and phosphorylate the latent cytoplasmic NFB/IB complex, which is ensued by the proteolysis of IB. The NFB p65 sub-unit is then released; it translocates to the nucleus, and binds the genes containing B sites which upregulate the expression of the gene products [4]. IB is essential to B cell survival [5]. Typically, NFB translocation in unseparated blood cells is measured by an electrophoretic gel mobility shift assay [6] or by molecular biology techniques [7]. Recently, a flow cytometry assay has been set up to allow rapid detection and quantification of DNA-bound NFB [8]. This method is particularly interesting for examining the capacity of certain molecules to induce cellular activation via the NFB pathway. This novel technique has been successfully adapted to monocyte-derived dendritic cells shortly stimulated with lipopolysaccharide [9]. As B lymphocytes are not only critically important cells in many aspects of the immune response, but may also be susceptible to genetic dysregulations leading to minor to most severe pathology, we have designed a new flow cytometry protocol to satisfactorily and reproducibly analyse NFB translocation after staining of isolated nuclei of purified tonsil B lymphocytes.
Materials and methods
Cell preparations
Tonsils (n=5) were obtained from individuals undergoing tonsillectomy for chronic tonsillitis. Tonsil Mononuclear Cells were prepared as described previously [10]. Peripheral blood B lymphocytes were obtained from Buffy coats prepared by the Regional Blood Bank in Saint-Etienne (France). They were isolated by gradient density centrifugation using Lymphoprep (Nycomed, Oslo, Norway). CD19+ B lymphocytes were purified by positive selection using anti-CD19-conjugated magnetic beads and a VarioMacs magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instruction with minor modifications [11]. Purity was routinely >98% as assessed by fluorescence analysis.
Cell stimulation
Purified CD19+ B cells were cultured in Iscove Dulbecco's Modified Medium (IDMM; Bio-Whittaker Europe, Verviers, Belgium) supplemented exactly as described previously [10]. B cell activation was performed using varying concentrations of a polyclonal B cell activator, i.e. anti-µ-Fab'2 fragments (Irvine Scientific, Santa Anna, CA, USA) at different time points.
Detection of NFB translocation by flow cytometric assay in CD19+ B cells nuclei preparations
CD19+ B cells were cultured at a concentration of 5×105 cells/ml per culture tube (Falcon, Le Pont de Claix, France), in the absence or presence of varying concentrations of anti-µ fragment Abs (from 0.1 to 1000 ng/ml) for 45 min at 37 °C. After incubation, B cells were washed with PBS containing 10% endotoxin-free FCS (BioMedia, Boussens, France). Nuclei were prepared by incubating the cells with 200 µl Pipes–Triton buffer (10 mM Pipes, 0.1 M NaCl, 2 mM MgCl2; Sigma–Aldrich, Steiheim, Germany and 0.1% Triton X 100; Boehringer Mannheim, Mannheim, Germany) in PBS for 30 min at 4 °C. After two washes in PBS–FCS, nuclei were stained with anti-NFB antibody at 5 µg/ml for 45 min at 4 °C. Mouse anti-human NFB p65 monoclonal antibody sc-8008 (Santa Cruz, CA, USA) recognizes epitopes mapping the C amino-acid terminus of human origin NFB p65. After two washes in PBS–FCS, nuclei were incubated for 45 min at 4 °C with FITC-conjugated goat anti-mouse anti-Ig antibody fragments (1/50; DAKO Glostrup, Denmark). After washings in PBS–FCS, nuclei were analysed by flow cytometry on a FACSvantage SE (BD Biosciences, San Jose, CA, USA) equipped with the pulse processor plus module, permitting the doublet discrimination analysis. The software used was CellQuest Pro (BD Biosciences). Nuclei were counterstained with 250 µg/ml propidium iodide (PI; Sigma–Aldrich). Single nuclei were gated on the basis of PI staining (FL-3 area measured at 630 nm), after doublet elimination by FL-3 area versus FL-3 width measurement. Voltage settings on FITC parameters (FL1 height measured at 530 nm) were done on isotype control samples. PI signals were acquired after linear amplification while FITC signals were acquired after logarithmic amplification. A total of 3×104 events were recorded for each sample. Successive experiments and analyses on purified tonsil B lymphocyte populations demonstrated reproducibility of the technique with limited inter-individual variation for a given culture condition (not shown).
Statistics
Inter-experiment comparisons, e.g. in stimulated versus unstimulated cells were analysed by means of the Wilcoxon paired test (Statview, SAS Inst., Cary, NC).
Results and discussion
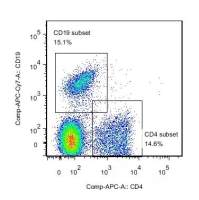
Control experiments ensured that NFB was located in the cytosol of unstimulated CD19+ B cells and exposure to a polyclonal B cell activator such as anti-µ-Fab'2 fragments caused the translocation of NFB into the nuclei in a dose- and time-dependent manner ( Fig. 1A and B). Intranuclear translocation of NFB p65 was induced after a 45 min stimulation with 0.1 to 10 ng/ml of anti-µ fragment Abs ( Fig. 1A). The highest signal was detected for a 10 ng/ml stimulus compared to the unstimulated condition (P<0.05). The signal declined significantly at higher concentrations of the stimulating antibodies (100 and 1000 ng/ml) compared to unstimulated cells (P<0.05). Ten nanograms per millilier of anti-µ fragment Abs were thus considered as the optimal stimulus for this and the following experiments. In terms of kinetics of NFB translocation the highest response was observed after a 45 min stimulation compared to as in unstimulated cells (P<0.05) with significant mean fluorescence intensity (MFI) decrease after a 45 min stimulation ( Fig. 1B). As can be seen in Fig. 2, purified CD19+ B cells—cultured at a concentration of 5×105 cells per tube in the presence of optimal concentrations of anti-µ fragment Abs (10 ng/ml) for 45 min at 37 °C—induced a mean 60% (range: 45–67%) MFI- and thus, nuclear NFB translocation-increase. As can also be seen in Fig. 2 ( Fig. 2A), there seems to be a dual profile of NFB activation when nuclei of purified B cells from tonsils were tested. This could be due to the existence of different B cell sub-populations within the tested cell population, as tonsils usually comprise of naïve and pre-switched B cells [10]. To test for this hypothesis, we assayed purified CD19+ B lymphocytes obtained from peripheral blood. Peripheral blood comprise chiefly of naïve B cells, with only a minority of pre-switched B lymphocytes [10]. As can be observed in Fig. 2B there is a one-pike profile of NFB staining. The two-pike profile of NFB staining observed in using tonsil B cells is thus very likely a consequence of the coexistence of at least a dual B cell population within this B cell source. The possibility of a differential response to the stimulus between supposedly naïve and non-naïve B cells has been recently raised by Bernasconi et al. [12].
It appears of critical interest to examine precisely the NFB activation pathway in B lymphocytes [2,5,13,14] , as B lymphocytes are not restricted to the function of secreting antibodies. Activated B cells are instrumental in the regulation of the all immune process, as evidenced by their central function is lymph node germinal centers [15]. B lymphocytes fall into the category of competent, active, and regulatory cells for the immune functions. Furthermore, B lymphocytes represent the main hematological cell subset being potentially subjected to dysfunction and genetic mutation leading to moderate, and even much more frequently, severe disease [16]. As this biological event—as others—still come from indirect evidence, there is a great need to explore further molecular events taking place in B cells during activation and differentiation at homeostasis and in pathology. The human blood B cells can be purified at homogeneity (98%). We thus aimed at examining NFB translocation during in vitro activation through the specific receptor for antigen using a technique convenient in most laboratories such as flow cytometry. This gave considerable advantages (feasibility, sensitivity, reproducibility, ease) over the measurement of NFB translocation by means of electrophoretic mobility shift assay [6], it is more accessible than confocal cytometry [9]. To the best of our knowledge, the flow cytometry analysis of NFB translocation in B cells was not as-yet adapted and made available for the scientific community. This technique now allows the analysis of signals delivered in vitro by different activation pathways such as antigen, CD40, Fc or cytokine receptors, or ex vivo in B cell pathology (malignancy).
Acknowledgements
The authors gratefully acknowledge the technical help of Audrey Granjon and Emilie Menessier. Fabrice Cognasse holds a fellowship from the Région Rhône-Alpes in partnership with Aventis-Pasteur Inc., Marcilly l'Etoile, France. They deeply thank Pr. C. Martin for providing them with tonsils and Drs. P. Chavarin and S. Acquart for providing them with Blood Buffy Coats. They are also grateful to Dr. T.A. Moore (Wichita, KS) and Dr. M Cogné (Limoges, France) for kindly revising this manuscript.
References
[[1]] May M.J. and Ghosh S. (1998)
Immunol. Today, 19:80-88. Full text MEDLINE Cited by
[[2]] Gugasyan R., Grumont R., Grossmann M., Nakamura Y., Pohl T., Nesic D. and Gerondakis S. (2000)
Immunol. Rev., 176:134-140. MEDLINE Cited by
[[3]] Umezawa K., Ariga A. and Matsumoto N. (2000)
Anticancer Drug Des., 15:239-244. MEDLINE Cited by
[[4]] Baldwin A.S. Jr. (1996)
Annu. Rev. Immunol., 14:649-683. MEDLINE Cited by
[[5]] Pasparakis M., Schmidt-Supprian M. and Rajewsky K. (2002)
J. Exp. Med., 196:743-752. Full text MEDLINE Cited by
[[6]] Liu J.L., Chiles T.C., Sen R.J. and Rothstein T.L. (1991)
J. Immunol., 146:1685-1691. MEDLINE Cited by
[[7]] Jobin C., Haskill S., Mayer L., Panja A. and Sartor R.B. (1997)
J. Immunol., 158:226-234. MEDLINE Cited by
[[8]] Foulds S. (1997)
Cytometry, 29:182-186. MEDLINE Cited by
[[9]] Blaecke A., Delneste Y., Herbault N., Jeannin P., Bonnefoy J.Y., Beck A. and Aubry J.P. (2002)
Cytometry, 48:71-79. MEDLINE Cited by
[[10]] Garraud O., Diouf A., Nguer C.M., Tall A., Marrama L. and Perraut R. (2001)
Scand. J. Immunol., 54:606-612. MEDLINE Cited by
[[11]] Cognasse F., Beniguel L., Neron-Bancel L., Sabido O., Genin C. and Garraud O. (2001)
Morphologie, 85:56.
[[12]] Bernasconi N.L., Traggiai E. and Lanzavecchia A. (2002)
Science, 298:2199-2202. Full text Cited by
[[13]] Ren H., Schmalstieg A., Yuan D. and Gaynor R.B. (2002)
J. Immunol., 168:577-587. MEDLINE Cited by
[[14]] Saijo K., Mecklenbrauker I., Santana A., Leitger M., Schmedt C. and Tarakhovsky A. (2002)
J. Exp. Med., 195:1647-1652. Full text MEDLINE Cited by
[[15]] Garraud O. and Nutman T. (1993)
Bull. Inst. Pasteur., 94:285-309. ScienceDirect Cited by
[[16]] Sanchez-Beato M., Sanchez-Aguilera A. and Piris M.A. (2003)
Blood, 101:1220-1235. Full text MEDLINE Cited by